
TURKU, FINLAND and BRUSSELS, BELGIUM
November 29, 2012
European Commission has granted €6 million from the seventh framework program (FP7) to support the FP-1201-lyo clinical phase III program (“Traumakine”), focusing to develop a first pharmacological treatment for acute respiratory distress syndrome (ARDS). The Consortium consists of the European Commission as a granting Agency, Faron Pharmaceuticals as a Coordinator and three other participating Partners of the Traumakine program, University College London Hospital (UCLH), University of Torino and University of Turku.
“We are very delighted for this grant approval by European Commission”, says Traumakine’s program Coordinator, Dr. Markku Jalkanen. “These funds cover only partially the whole Traumakine-program but is a significant recognition of this orphan drug program by EU as only 10 % of the applications were approved”, continues Jalkanen.
The key activity of the Traumakine-program is a phase III clinical study aiming at the European marketing authorization of FP-1201-lyo treatment of ARDS. The previously performed clinical study with FP-1201 has indicated significant decrease in mortality of the ALI/ARDS patients with good safety profile. The phase III trial will be a pan-European study conducted by more than 100 hospitals with significant intensive care units (ICU) around Europe. Faron has been granted an orphan drug status for the treatment of ALI/ARDS with interferon-beta by European Commission and European Medicines Agency (EMA) under the registration number EU/3/07/505.
About ALI/ARDS and FP-1201 (human recombinant interferon-beta 1a)
ALI and ARDS are serious clinical disorders, which follow a variety of severe direct and indirect lung insults. In serious life threatening situations such as infection leading to sepsis or trauma causing massive tissue injury, an escalation of the systemic inflammatory response leads to multiple organ failure including ALI/ARDS. In the case of ALI/ARDS the predominant patho-physiological result is increased vascular leakage, which has been shown to be due to the lack of adenosine, an end product of AMP degradation by 5’-nucleotidase (CD73). Adenosine acts to enhance endothelial barrier function via adenosine receptor activation. Therefore, any biological substance, which acts to increase adenosine level, will reduce vascular leakage and be of benefit in ALI/ARDS patients. Such substances are type I interferons, and especially the interferon-beta (IFN-beta). IFN-beta has been shown to up-regulate 5’-nucleotidase (also known as a CD73 molecule and expressed abundantly by normal endothelial cells) and prevent ALI in animal models (Kiss et al. (2007) Eur. J. Immunol. 37:3334). IFN-beta is therefore a potential treatment for ALI/ARDS. The schematic drawing below (Figure 1) illustrates this principle.
The medical need for an effective and safe treatment of ALI/ARDS is high, since this condition is life threatening with 35-45 % mortality rate (Rubenfeld et al. (2005) N Engl J Med 353, 1685) (Phua et al. (2009) Am J Respir Crit Care Med 179, 220) and affects close to 200.000 patients in Europe alone. Currently no approved pharmacological treatment of ALI/ARDS by the regulatory authorities in Europe (EMA) or in the USA (FDA) is available. The final product for the market is a lyophilized intravenous formulation of IFN-beta and is called FP-1201-lyo.
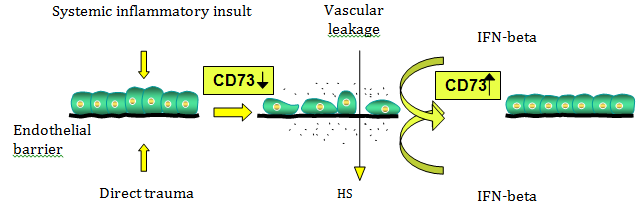
Figure 1: A model of IFN-beta action in acute lung injuries and prevention of vascular leakage. The injury-induced loss of endothelial barrier results in leakage of protein-rich fluid (HSA) into lung space, restricting/preventing respiration and leading to ARDS.
For more information on the Traumakine Program and Consortium, please visit: https://www.traumakine.eu or contact:
Dr. Markku Jalkanen, Coordinator for the Traumakine program
Phone: +358-40-520-6124
E-mail: markku.jalkanen@faronpharmaceuticals.com